Write the Ground-state Electron Configurations of the Following Ions. (a) Li+
3.1: Electron Configurations (Problems)
-
- Last updated
- Save as PDF
- Page ID
- 119830
PROBLEM \(\PageIndex{1}\)
Using complete subshell notation (no abbreviations), predict the electron configuration of each of the following atoms:
- C
- P
- V
- Sb
- Sm
- Answer a
-
1s22s22p2
- Answer b
-
1s22s22p63s23p3
- Answer c
-
1s22s22p63s23p63d34s2
- Answer d
-
1s22s22p63s23p63d104s24p64d105s25p3
- Answer e
-
1s22s22p63s23p63d104s24p64d104f65s25p66s2.
PROBLEM \(\PageIndex{2}\)
Using complete subshell notation, predict the electron configuration of each of the following atoms:
- N
- Si
- Fe
- Te
- Tb
- Answer a
-
1s 22s 22p 3
- Answer b
-
1s 22s 22p 63s 23p 2
- Answer c
-
1s 22s 22p 63s 23p 64s 23d 6
- Answer d
-
1s 22s 22p 63s 23p 64s 23d 104p 65s 24d 105p 4
- Answer e
-
1s 22s 22p 63s 23p 64s 23d 104p 65s 24d 105p 66s 24f 9
PROBLEM \(\PageIndex{3}\)
Use an orbital diagram to describe the electron configuration of the valence shell of each of the following atoms:
- N
- Si
- Fe
- Te
- Mo
- Answer a
-
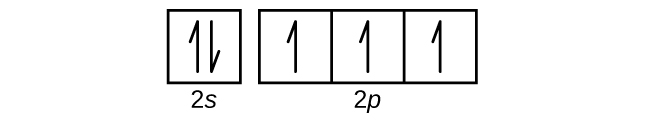
- Answer b
-
- Answer c
-
- Answer d
-
- Answer e
-
PROBLEM \(\PageIndex{4}\)
Using complete subshell notation (1s 22s 22p 6, and so forth), predict the electron configurations of the following ions.
- N3–
- Ca2+
- S–
- Cs2+
- Cr2+
- Gd3+
- Answer a
-
1s 22s 22p 6
- Answer b
-
1s 22s 22p 63s 23p 6
- Answer c
-
1s 22s 22p 63s 23p 5
- Answer d
-
1s 22s 22p 63s 23p 64s 23d 104p 65s 24d 105p 5
- Answer e
-
1s 22s 22p 63s 23p 64s 23d 2
- Answer f
-
1s 22s 22p 63s 23p 64s 23d 104p 65s 24d 105p 66s 24f 5
- Click here to see a video of the solution
PROBLEM \(\PageIndex{5}\)
Which atom has the electron configuration: 1s 22s 22p 63s 23p 64s 23d 104p 65s 24d 2?
- Answer
-
Zr
PROBLEM \(\PageIndex{6}\)
Which atom has the electron configuration: 1s 22s 22p 63s 23p 63d 74s 2?
- Answer
-
Co
PROBLEM \(\PageIndex{7}\)
a. Which ion with a +1 charge has the electron configuration 1s 22s 22p 63s 23p 63d 104s 24p 6?
b. Which ion with a –2 charge has this configuration?
- Answer a
-
Rb+
- Answer b
-
Se2−
PROBLEM \(\PageIndex{8}\)
Which of the following atoms contains only three valence electrons: Li, B, N, F, Ne?
- Answer
-
B
PROBLEM \(\PageIndex{9}\)
Which of the following has two unpaired electrons?
- Mg
- Si
- S
- Both Mg and S
- Both Si and S.
- Answer
-
Although both (b) and (c) are correct, (e) encompasses both and is the best answer.
PROBLEM \(\PageIndex{10}\)
Which atom would be expected to have a half-filled 6p subshell?
- Answer
-
Bi
PROBLEM \(\PageIndex{11}\)
Which atom would be expected to have a half-filled 4s subshell?
- Answer
-
K
PROBLEM \(\PageIndex{12}\)
In one area of Australia, the cattle did not thrive despite the presence of suitable forage. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Cobalt forms cations in two oxidation states, Co2+ and Co3+. Write the electron structure of the two cations.
- Answer
-
Co2 +: 1s 22s 22p 63s 23p 64s 23d 5
Co3 +: 1s 22s 22p 63s 23p 64s 23d 4
- Click here to see a video of the solution
PROBLEM \(\PageIndex{13}\)
Thallium was used as a poison in the Agatha Christie mystery story "The Pale Horse." Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. Write the electron structure of the +1 cation of thallium.
- Answer
-
1s 22s 22p 63s 23p 63d 104s 24p 64d 105s 25p 66s 24f 145d 10
PROBLEM \(\PageIndex{14}\)
Write the electron configurations for the following atoms or ions:
- B3+
- O–
- Cl3+
- Ca2+
- Ti
- Answer a
-
1s 2
- Answer b
-
1s 22s 22p 5
- Answer c
-
1s 22s 22p 63s 23p 2
- Answer d
-
1s 22s 22p 63s 23p 6
- Answer e
-
1s 22s 22p 63s 23p 64s 23d 2
PROBLEM \(\PageIndex{15}\)
Cobalt–60 and iodine–131 are radioactive isotopes commonly used in nuclear medicine. How many protons, neutrons, and electrons are in atoms of these isotopes? Write the complete electron configuration for each isotope.
- Answer
-
Co has 27 protons, 27 electrons, and 33 neutrons: 1s 22s 22p 63s 23p 64s 23d 7.
I has 53 protons, 53 electrons, and 78 neutrons: 1s 22s 22p 63s 23p 63d 104s 24p 64d 105s 25p 5.
PROBLEM \(\PageIndex{16}\)
Atoms of which group in the periodic table have a valence shell electron configuration of ns 2 np 3?
- Answer
-
15 (5A)
PROBLEM \(\PageIndex{17}\)
Atoms of which group in the periodic table have a valence shell electron configuration of ns 2?
- Answer
-
2 (2A)
PROBLEM \(\PageIndex{18}\)
Does a cation gain protons to form a positive charge or does it lose electrons?
- Answer
-
The protons in the nucleus do not change during normal chemical reactions. Only the outer electrons move. Positive charges form when electrons are lost.
PROBLEM \(\PageIndex{19}\)
Iron(III) sulfate [Fe2(SO4)3] is composed of Fe3+ and \(\ce{SO4^2-}\) ions. Explain why a sample of iron(III) sulfate is uncharged.
- Answer
-
Two cations with a +3 charge give a total of +6 charge, while three anions of -2 charge give a total of -6 charge. +6-6=0, so when these ions bond, the charges will cancel, leaving the resulting compound uncharged.
PROBLEM \(\PageIndex{20}\)
Which of the following atoms would be expected to form negative ions in binary ionic compounds and which would be expected to form positive ions: P, I, Mg, Cl, In, Cs, O, Pb, Co?
- Answer
-
P, I, Cl, and O would form anions because they are nonmetals. Mg, In, Cs, Pb, and Co would form cations because they are metals.
PROBLEM \(\PageIndex{21}\)
Which of the following atoms would be expected to form negative ions in binary ionic compounds and which would be expected to form positive ions: Br, Ca, Na, N, F, Al, Sn, S, Cd?
- Answer
-
Anions: Br, N, F, S,
Cations: Ca, Na, Al, Sn (because it's a metal), Cd (because it is a metal)
PROBLEM \(\PageIndex{22}\)
Predict the charge on the monatomic ions formed from the following atoms in binary ionic compounds:
- P
- Mg
- Al
- O
- Cl
- Cs
- Answer a
-
P3–
- Answer b
-
Mg2+
- Answer c
-
Al3+
- Answer d
-
O2–
- Answer e
-
Cl–
- Answer f
-
Cs+
PROBLEM \(\PageIndex{23}\)
Predict the charge on the monatomic ions formed from the following atoms in binary ionic compounds:
a. I
b. Sr
c. K
d. N
e. S
f. In
- Answer a
-
I-
- Answer b
-
Sr2+
- Answer c
-
K+
- Answer d
-
N3-
- Answer e
-
S2-
- Answer f
-
In3+
PROBLEM \(\PageIndex{24}\)
Write the noble gas electron configuration for each of the following ions:
a. As3–
b. I–
c. Be2+
d. Cd2+
e. O2–
f. Ga3+
g. Li+
h. N3–
i. Sn2+
j. Co2+
k. Fe2+
l. As3+
- Answer a
-
[Ar]4s 23d 104p 6
- Answer b
-
[Kr]4d 105s 25p 6
- Answer c
-
1s 2
- Answer d
-
[Kr]4d 10
- Answer e
-
[He]2s 22p 6
- Answer f
-
[Ar]3d 10
- Answer g
-
1s 2
- Answer h
-
[He]2s 22p 6
- Answer i
-
[Kr]4d 105s 2
- Answer j
-
[Ar]3d 7
- Answer k
-
[Ar]3d 6
- Answer l
-
[Ar]3d 104s 2
PROBLEM \(\PageIndex{25}\)
Write out the full electron configuration for each of the following atoms and for the monatomic ion found in binary ionic compounds containing the element:
- Al
- Br
- Sr
- Li
- As
- S
- Answer a
-
Al: 1s 22s 22p 63s 23p 1
Al3+: 1s 22s 22p 6
- Answer b
-
Br: 1s 22s 22p 63s 23p 63d 104s 24p 5
Br-: 1s 22s 22p 63s 23p 63d 104s 24p 6
- Answer c
-
Sr: 1s 22s 22p 63s 23p 63d 104s 24p 65s 2
Sr2+: 1s 22s 22p 63s 23p 63d 104s 24p 6
- Answer d
-
Li: 1s 22s 1
Li+: 1s 2
- Answer e
-
As: 1s 22s 22p 63s 23p 63d 104s 24p 3
As3 -: 1s 22s 22p 63s 23p 63d 104s 24p 6
- Answer f
-
S: 1s 22s 22p 63s 23p 4
S2-: 1s 22s 22p 63s 23p 6
Feedback
Think one of the answers above is wrong? Let us know here.
Write the Ground-state Electron Configurations of the Following Ions. (a) Li+
Source: https://chem.libretexts.org/Courses/Oregon_Institute_of_Technology/OIT%3A_CHE_202_-_General_Chemistry_II/Unit_3%3A_Periodic_Patterns/3.1%3A_Electron_Configurations/3.1%3A_Electron_Configurations_(Problems)